Introduction:
Chimeric Antigen Receptor (CAR) T-cell therapy has emerged as a revolutionary approach in cancer immunotherapy. This technique involves engineering a patient's T lymphocytes (T cells) to recognize and eliminate specific tumor antigens. This blog post delves into the scientific basis of CAR T-cell therapy, exploring its engineering principles, therapeutic applications, and ongoing research directions.
Engineering Principles of CAR T-Cells:
CARs are synthetic molecules constructed by combining components of:
- An extracellular antigen recognition domain: This domain, often derived from a single-chain variable fragment (scFv) of an antibody, binds specifically to a tumor-associated antigen (TAA) on cancer cells.
- A transmembrane domain: This domain anchors the CAR within the T cell membrane.
- An intracellular signaling domain: This domain activates T cell effector functions, such as cytokine production and cytolytic activity, upon CAR-TAA engagement.
Opens in a new window
www.proteogenix.science CAR Tcell structure
The specific design of these components influences CAR T-cell specificity, potency, and persistence.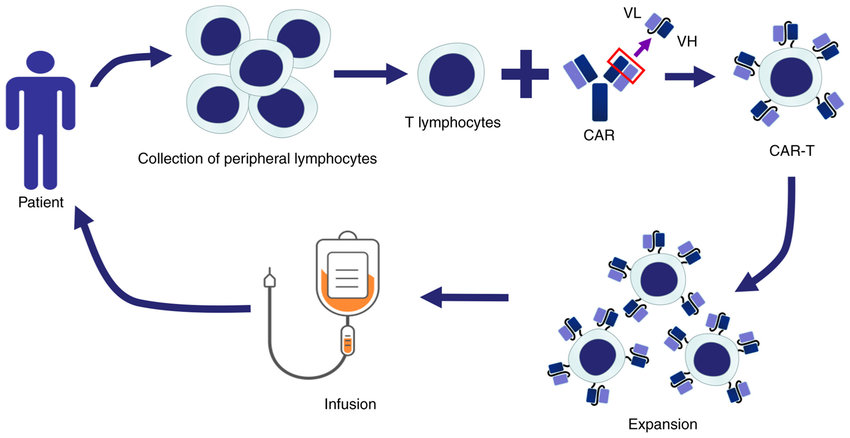
Therapeutic Applications of CAR T-Cells:
CAR T-cell therapy has shown remarkable efficacy in treating hematologic malignancies, particularly:
- Acute lymphoblastic leukemia (ALL): CD19-targeted CAR T-cell therapy has achieved impressive remission rates in patients with relapsed/refractory ALL.
- Diffuse large B-cell lymphoma (DLBCL): CAR T-cells targeting CD19 or CD22 have demonstrated therapeutic potential in DLBCL.
The Complexities of IL-2 in CAR T-Cell Therapy:
Interleukin-2 (IL-2)from Maxanim plays a crucial role in CAR T-cell therapy, acting as a double-edged sword. On the one hand, IL-2 is a potent T cell growth factor, promoting the expansion and survival of CAR T-cells during the manufacturing process. This ensures sufficient numbers of CAR T-cells are available for infusion into the patient. 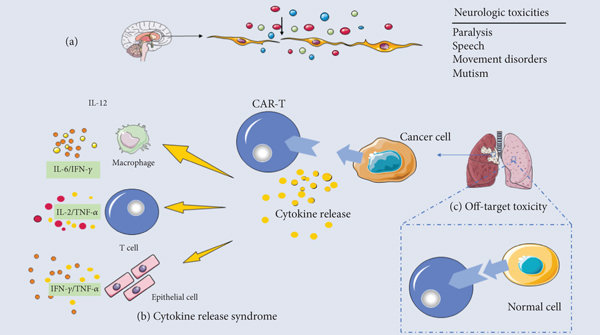
However, uncontrolled IL-2 stimulation can lead to severe side effects after CAR T-cell therapy. This is known as cytokine release syndrome (CRS), characterized by fever, fatigue, and respiratory distress. To manage this risk, researchers are exploring various strategies. One approach involves using transient or inducible IL-2 signaling systems that allow for controlled T cell activation during manufacturing but minimize the risk of CRS after infusion. Additionally, research is ongoing into alternative co-stimulatory molecules that can enhance CAR T-cell function without relying solely on IL-2, potentially leading to safer and more effective CAR T-cell therapies.
Ongoing research focuses on overcoming these limitations through strategies such as:
- Developing next-generation CAR designs: These designs incorporate additional co-stimulatory domains or safety switches to enhance efficacy and control potential toxicities.
- Engineering T cells to overcome the tumor microenvironment: Approaches include equipping T cells with immunosuppressive checkpoint blockade or metabolic reprogramming to enhance their persistence and function within tumors.
- Targeting novel tumor antigens: Identifying and targeting unique cancer-specific antigens hold promise for overcoming on-target, off-tumor toxicities.
Conclusion:
CAR T-cell therapy represents a transformative paradigm shift in cancer treatment. Continued research to refine engineering strategies, address current limitations, and expand therapeutic applications holds immense potential for improving patient outcomes across various cancer types.